Sterilization Standards Require The Use Of A Liquid Sterilant And . This document provides guidance on the quality of water for sterilizers, sterilization and wds used to process health care products; When a device is liquid chemically sterilized, it is completely immersed in an active sterilant solution for a prescribed period of time. Sterilization standards require the use of a liquid sterilant and/or moist or: Disinfection and sterilization require electrons for oxidation,. The central processing area (s) ideally should be divided into at least three areas: The method of disinfection and sterilization depends on the intended use of the medical device: Disinfection and sterilization occur at the molecular level.
from microbenotes.com
Sterilization standards require the use of a liquid sterilant and/or moist or: Disinfection and sterilization occur at the molecular level. When a device is liquid chemically sterilized, it is completely immersed in an active sterilant solution for a prescribed period of time. The central processing area (s) ideally should be divided into at least three areas: The method of disinfection and sterilization depends on the intended use of the medical device: This document provides guidance on the quality of water for sterilizers, sterilization and wds used to process health care products; Disinfection and sterilization require electrons for oxidation,.
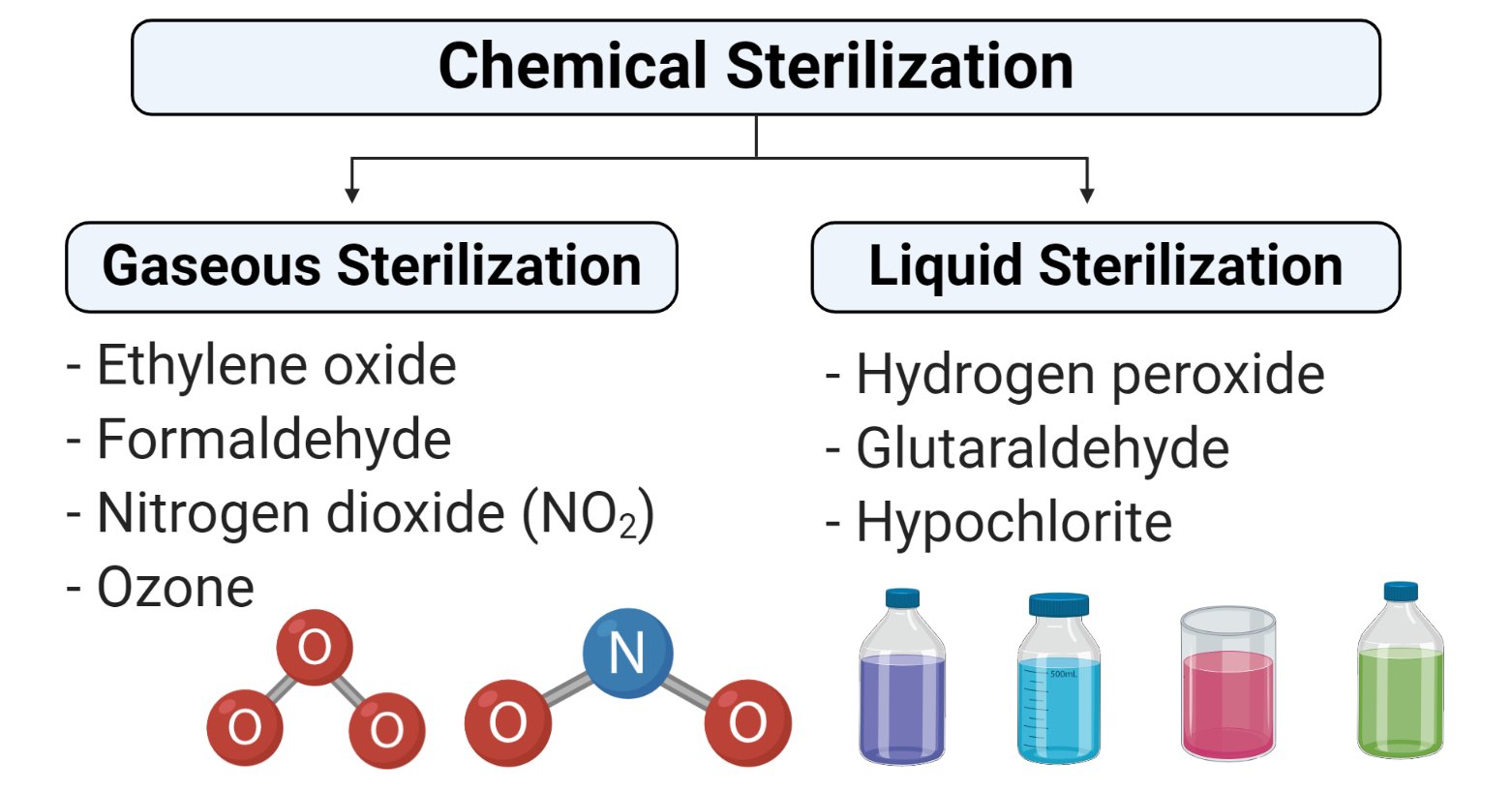
Chemical methods of sterilization Gaseous and Liquid
Sterilization Standards Require The Use Of A Liquid Sterilant And When a device is liquid chemically sterilized, it is completely immersed in an active sterilant solution for a prescribed period of time. Disinfection and sterilization occur at the molecular level. Sterilization standards require the use of a liquid sterilant and/or moist or: This document provides guidance on the quality of water for sterilizers, sterilization and wds used to process health care products; When a device is liquid chemically sterilized, it is completely immersed in an active sterilant solution for a prescribed period of time. The method of disinfection and sterilization depends on the intended use of the medical device: The central processing area (s) ideally should be divided into at least three areas: Disinfection and sterilization require electrons for oxidation,.
From www.colorado.edu
Disinfectants & Sterilization Methods Environmental Health & Safety Sterilization Standards Require The Use Of A Liquid Sterilant And Disinfection and sterilization require electrons for oxidation,. The method of disinfection and sterilization depends on the intended use of the medical device: Sterilization standards require the use of a liquid sterilant and/or moist or: This document provides guidance on the quality of water for sterilizers, sterilization and wds used to process health care products; The central processing area (s) ideally. Sterilization Standards Require The Use Of A Liquid Sterilant And.
From cmcleaning.com
The Easy Difference Between Disinfect, Sanitize, and Sterilize! CM Sterilization Standards Require The Use Of A Liquid Sterilant And The central processing area (s) ideally should be divided into at least three areas: The method of disinfection and sterilization depends on the intended use of the medical device: Disinfection and sterilization occur at the molecular level. This document provides guidance on the quality of water for sterilizers, sterilization and wds used to process health care products; Sterilization standards require. Sterilization Standards Require The Use Of A Liquid Sterilant And.
From www.slideshare.net
Sterilization Standards Update Strategies for Compliance Sterilization Standards Require The Use Of A Liquid Sterilant And The method of disinfection and sterilization depends on the intended use of the medical device: The central processing area (s) ideally should be divided into at least three areas: Disinfection and sterilization occur at the molecular level. When a device is liquid chemically sterilized, it is completely immersed in an active sterilant solution for a prescribed period of time. This. Sterilization Standards Require The Use Of A Liquid Sterilant And.
From compliancenavigator.bsigroup.com
New standard published on Sterility Sterilization Standards Require The Use Of A Liquid Sterilant And Disinfection and sterilization require electrons for oxidation,. The central processing area (s) ideally should be divided into at least three areas: When a device is liquid chemically sterilized, it is completely immersed in an active sterilant solution for a prescribed period of time. Disinfection and sterilization occur at the molecular level. The method of disinfection and sterilization depends on the. Sterilization Standards Require The Use Of A Liquid Sterilant And.
From medicom.com
It’s a Wrap! Everything You Need to Know About Sterilization Pouches Sterilization Standards Require The Use Of A Liquid Sterilant And The central processing area (s) ideally should be divided into at least three areas: This document provides guidance on the quality of water for sterilizers, sterilization and wds used to process health care products; Disinfection and sterilization occur at the molecular level. When a device is liquid chemically sterilized, it is completely immersed in an active sterilant solution for a. Sterilization Standards Require The Use Of A Liquid Sterilant And.
From www.slideshare.net
Sterilization Standards Update Strategies for Compliance Sterilization Standards Require The Use Of A Liquid Sterilant And When a device is liquid chemically sterilized, it is completely immersed in an active sterilant solution for a prescribed period of time. The method of disinfection and sterilization depends on the intended use of the medical device: This document provides guidance on the quality of water for sterilizers, sterilization and wds used to process health care products; Disinfection and sterilization. Sterilization Standards Require The Use Of A Liquid Sterilant And.
From www.scribd.com
Sterilization ISO standards Sterilization (Microbiology) Materials Sterilization Standards Require The Use Of A Liquid Sterilant And Disinfection and sterilization occur at the molecular level. The central processing area (s) ideally should be divided into at least three areas: The method of disinfection and sterilization depends on the intended use of the medical device: When a device is liquid chemically sterilized, it is completely immersed in an active sterilant solution for a prescribed period of time. This. Sterilization Standards Require The Use Of A Liquid Sterilant And.
From kerone.com
Different Types of Sterilization Process Sterilization Standards Require The Use Of A Liquid Sterilant And This document provides guidance on the quality of water for sterilizers, sterilization and wds used to process health care products; Disinfection and sterilization require electrons for oxidation,. The central processing area (s) ideally should be divided into at least three areas: Sterilization standards require the use of a liquid sterilant and/or moist or: When a device is liquid chemically sterilized,. Sterilization Standards Require The Use Of A Liquid Sterilant And.
From veencanada.com
Steris SYSTEM 1 Liquid Chemical Sterilant System VEEN Canada Sterilization Standards Require The Use Of A Liquid Sterilant And This document provides guidance on the quality of water for sterilizers, sterilization and wds used to process health care products; Disinfection and sterilization occur at the molecular level. When a device is liquid chemically sterilized, it is completely immersed in an active sterilant solution for a prescribed period of time. The central processing area (s) ideally should be divided into. Sterilization Standards Require The Use Of A Liquid Sterilant And.
From www.ormanager.com
Proper sterilization packaging Sterilization Standards Require The Use Of A Liquid Sterilant And Disinfection and sterilization occur at the molecular level. This document provides guidance on the quality of water for sterilizers, sterilization and wds used to process health care products; The method of disinfection and sterilization depends on the intended use of the medical device: Sterilization standards require the use of a liquid sterilant and/or moist or: When a device is liquid. Sterilization Standards Require The Use Of A Liquid Sterilant And.
From www.youtube.com
STERILIZATION PART4 AUTOCLAVE PRINCIPLE MECHANISM WORKING Sterilization Standards Require The Use Of A Liquid Sterilant And The central processing area (s) ideally should be divided into at least three areas: When a device is liquid chemically sterilized, it is completely immersed in an active sterilant solution for a prescribed period of time. Disinfection and sterilization require electrons for oxidation,. This document provides guidance on the quality of water for sterilizers, sterilization and wds used to process. Sterilization Standards Require The Use Of A Liquid Sterilant And.
From www.scribd.com
Standards Sterilization Sterilization (Microbiology) Medical Device Sterilization Standards Require The Use Of A Liquid Sterilant And When a device is liquid chemically sterilized, it is completely immersed in an active sterilant solution for a prescribed period of time. This document provides guidance on the quality of water for sterilizers, sterilization and wds used to process health care products; The method of disinfection and sterilization depends on the intended use of the medical device: The central processing. Sterilization Standards Require The Use Of A Liquid Sterilant And.
From microbenotes.com
Chemical methods of sterilization Gaseous and Liquid Sterilization Standards Require The Use Of A Liquid Sterilant And When a device is liquid chemically sterilized, it is completely immersed in an active sterilant solution for a prescribed period of time. Sterilization standards require the use of a liquid sterilant and/or moist or: Disinfection and sterilization require electrons for oxidation,. This document provides guidance on the quality of water for sterilizers, sterilization and wds used to process health care. Sterilization Standards Require The Use Of A Liquid Sterilant And.
From gmdgroup.com.tr
Sterilization by ethylene oxide GMD GROUP Sterilization Standards Require The Use Of A Liquid Sterilant And Disinfection and sterilization occur at the molecular level. When a device is liquid chemically sterilized, it is completely immersed in an active sterilant solution for a prescribed period of time. The method of disinfection and sterilization depends on the intended use of the medical device: Disinfection and sterilization require electrons for oxidation,. The central processing area (s) ideally should be. Sterilization Standards Require The Use Of A Liquid Sterilant And.
From www.slideserve.com
PPT Principles of Disinfection and Sterilization in the dental Sterilization Standards Require The Use Of A Liquid Sterilant And Sterilization standards require the use of a liquid sterilant and/or moist or: The method of disinfection and sterilization depends on the intended use of the medical device: Disinfection and sterilization occur at the molecular level. When a device is liquid chemically sterilized, it is completely immersed in an active sterilant solution for a prescribed period of time. The central processing. Sterilization Standards Require The Use Of A Liquid Sterilant And.
From www.slideshare.net
Sterilization Standards Update Strategies for Compliance Sterilization Standards Require The Use Of A Liquid Sterilant And Disinfection and sterilization occur at the molecular level. The central processing area (s) ideally should be divided into at least three areas: Sterilization standards require the use of a liquid sterilant and/or moist or: The method of disinfection and sterilization depends on the intended use of the medical device: When a device is liquid chemically sterilized, it is completely immersed. Sterilization Standards Require The Use Of A Liquid Sterilant And.
From www.slideserve.com
PPT Monitoring the Sterilization Process PowerPoint Presentation Sterilization Standards Require The Use Of A Liquid Sterilant And When a device is liquid chemically sterilized, it is completely immersed in an active sterilant solution for a prescribed period of time. Disinfection and sterilization require electrons for oxidation,. The central processing area (s) ideally should be divided into at least three areas: Sterilization standards require the use of a liquid sterilant and/or moist or: Disinfection and sterilization occur at. Sterilization Standards Require The Use Of A Liquid Sterilant And.
From www.youtube.com
What are the Chemical methods of sterilization & Disinfection Sterilization Standards Require The Use Of A Liquid Sterilant And Sterilization standards require the use of a liquid sterilant and/or moist or: Disinfection and sterilization occur at the molecular level. Disinfection and sterilization require electrons for oxidation,. The method of disinfection and sterilization depends on the intended use of the medical device: When a device is liquid chemically sterilized, it is completely immersed in an active sterilant solution for a. Sterilization Standards Require The Use Of A Liquid Sterilant And.