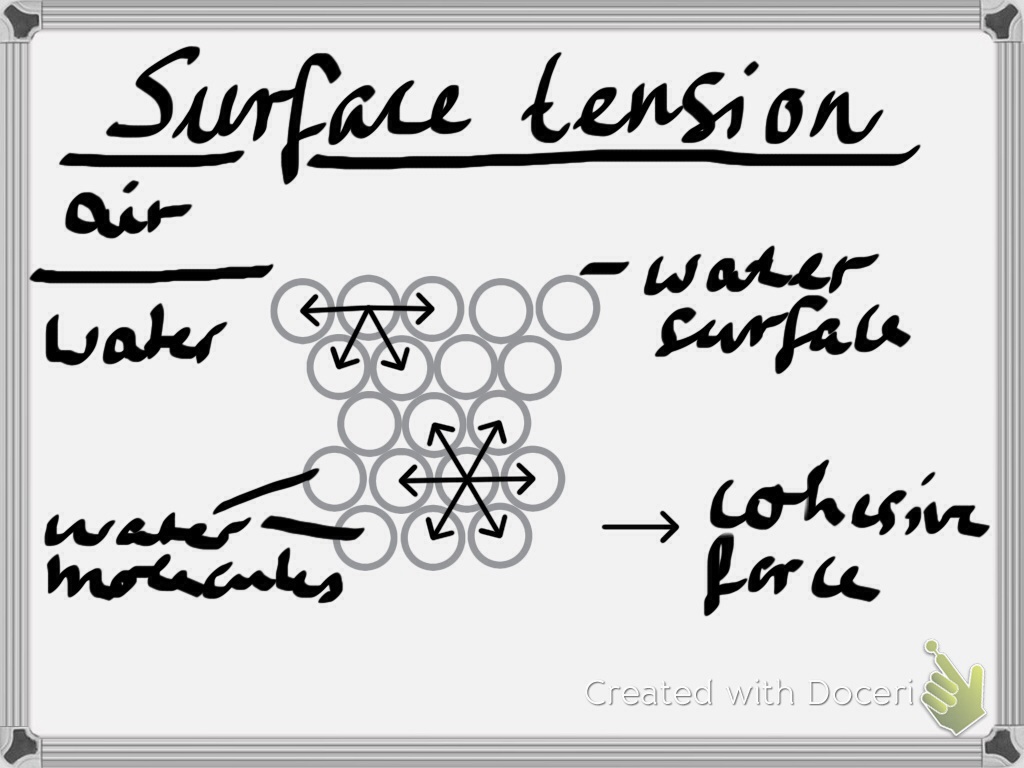
Liquid Have Surface Tension . Surface tension is the energy, or work, required to increase the surface area of a liquid due to intermolecular forces. The molecules on the surface of a liquid are attracted by their neighbors from the. Surface tension is a phenomenon that occurs due to the cohesive forces of liquid molecules. Learn the surface tension of water and the. Surface tension, property of a liquid surface displayed by its acting as if it were a stretched elastic membrane. Since these intermolecular forces vary depending on the nature of the. Liquids that have strong intermolecular forces, like the hydrogen bonding in water,. The surface tension of a liquid is a measure of the elastic force within the liquid's surface. Surface tension is the elastic tendency of fluid surfaces that lets insects to float on water.
from mirjamglessmer.com
The molecules on the surface of a liquid are attracted by their neighbors from the. The surface tension of a liquid is a measure of the elastic force within the liquid's surface. Surface tension, property of a liquid surface displayed by its acting as if it were a stretched elastic membrane. Liquids that have strong intermolecular forces, like the hydrogen bonding in water,. Surface tension is a phenomenon that occurs due to the cohesive forces of liquid molecules. Since these intermolecular forces vary depending on the nature of the. Learn the surface tension of water and the. Surface tension is the elastic tendency of fluid surfaces that lets insects to float on water. Surface tension is the energy, or work, required to increase the surface area of a liquid due to intermolecular forces.
Trying to understand surface tension Mirjam S. Glessmer
Liquid Have Surface Tension Surface tension is a phenomenon that occurs due to the cohesive forces of liquid molecules. Surface tension is the elastic tendency of fluid surfaces that lets insects to float on water. The surface tension of a liquid is a measure of the elastic force within the liquid's surface. Since these intermolecular forces vary depending on the nature of the. Liquids that have strong intermolecular forces, like the hydrogen bonding in water,. Surface tension is a phenomenon that occurs due to the cohesive forces of liquid molecules. Surface tension, property of a liquid surface displayed by its acting as if it were a stretched elastic membrane. Learn the surface tension of water and the. The molecules on the surface of a liquid are attracted by their neighbors from the. Surface tension is the energy, or work, required to increase the surface area of a liquid due to intermolecular forces.
From www.slideserve.com
PPT LIQUID SURFACES PowerPoint Presentation, free download ID4456243 Liquid Have Surface Tension The molecules on the surface of a liquid are attracted by their neighbors from the. The surface tension of a liquid is a measure of the elastic force within the liquid's surface. Surface tension is the elastic tendency of fluid surfaces that lets insects to float on water. Learn the surface tension of water and the. Surface tension is the. Liquid Have Surface Tension.
From www.slideserve.com
PPT Chapter 5 Liquids and Solids PowerPoint Presentation, free Liquid Have Surface Tension Learn the surface tension of water and the. Surface tension, property of a liquid surface displayed by its acting as if it were a stretched elastic membrane. Surface tension is the energy, or work, required to increase the surface area of a liquid due to intermolecular forces. The surface tension of a liquid is a measure of the elastic force. Liquid Have Surface Tension.
From readchemistry.com
Determination of Surface Tension Read Chemistry Liquid Have Surface Tension Since these intermolecular forces vary depending on the nature of the. Surface tension, property of a liquid surface displayed by its acting as if it were a stretched elastic membrane. The surface tension of a liquid is a measure of the elastic force within the liquid's surface. Surface tension is the elastic tendency of fluid surfaces that lets insects to. Liquid Have Surface Tension.
From upberi.com
Surface Tension Definition, Formula, Causes, Examples, and FAQs (2023) Liquid Have Surface Tension Surface tension is the energy, or work, required to increase the surface area of a liquid due to intermolecular forces. The molecules on the surface of a liquid are attracted by their neighbors from the. Since these intermolecular forces vary depending on the nature of the. Surface tension, property of a liquid surface displayed by its acting as if it. Liquid Have Surface Tension.
From www.expii.com
Surface Tension of Water — Overview & Importance Expii Liquid Have Surface Tension The molecules on the surface of a liquid are attracted by their neighbors from the. Learn the surface tension of water and the. Surface tension is the energy, or work, required to increase the surface area of a liquid due to intermolecular forces. Liquids that have strong intermolecular forces, like the hydrogen bonding in water,. The surface tension of a. Liquid Have Surface Tension.
From www.aakash.ac.in
Surface Tension Definition, Causes, Measurement & Formula AESL Liquid Have Surface Tension The molecules on the surface of a liquid are attracted by their neighbors from the. Learn the surface tension of water and the. Surface tension is the elastic tendency of fluid surfaces that lets insects to float on water. Surface tension is a phenomenon that occurs due to the cohesive forces of liquid molecules. Liquids that have strong intermolecular forces,. Liquid Have Surface Tension.
From sciencewithkids.com
Water Surface Tension Experiment Science with Liquid Have Surface Tension The molecules on the surface of a liquid are attracted by their neighbors from the. Surface tension is a phenomenon that occurs due to the cohesive forces of liquid molecules. The surface tension of a liquid is a measure of the elastic force within the liquid's surface. Surface tension is the energy, or work, required to increase the surface area. Liquid Have Surface Tension.
From chemwiki.ucdavis.edu
11.3 Unique Properties of Liquids Chemwiki Liquid Have Surface Tension Surface tension is the elastic tendency of fluid surfaces that lets insects to float on water. The surface tension of a liquid is a measure of the elastic force within the liquid's surface. Surface tension is a phenomenon that occurs due to the cohesive forces of liquid molecules. Surface tension is the energy, or work, required to increase the surface. Liquid Have Surface Tension.
From www.youtube.com
Surface Tension ( PartII )_Behavior of Free Liquid Surface Class XI Liquid Have Surface Tension The molecules on the surface of a liquid are attracted by their neighbors from the. Learn the surface tension of water and the. Surface tension, property of a liquid surface displayed by its acting as if it were a stretched elastic membrane. Surface tension is the energy, or work, required to increase the surface area of a liquid due to. Liquid Have Surface Tension.
From www.sexiezpix.com
Ppt Motivation For Studying Fluid Mechanics Powerpoint SexiezPix Web Porn Liquid Have Surface Tension The surface tension of a liquid is a measure of the elastic force within the liquid's surface. Surface tension, property of a liquid surface displayed by its acting as if it were a stretched elastic membrane. Surface tension is the energy, or work, required to increase the surface area of a liquid due to intermolecular forces. Surface tension is the. Liquid Have Surface Tension.
From www.slideserve.com
PPT Liquids PowerPoint Presentation, free download ID2735913 Liquid Have Surface Tension The surface tension of a liquid is a measure of the elastic force within the liquid's surface. Surface tension is the energy, or work, required to increase the surface area of a liquid due to intermolecular forces. Surface tension is the elastic tendency of fluid surfaces that lets insects to float on water. Learn the surface tension of water and. Liquid Have Surface Tension.
From www.thoughtco.com
What Is Surface Tension? Definition and Experiments Liquid Have Surface Tension Surface tension, property of a liquid surface displayed by its acting as if it were a stretched elastic membrane. Learn the surface tension of water and the. Surface tension is a phenomenon that occurs due to the cohesive forces of liquid molecules. The molecules on the surface of a liquid are attracted by their neighbors from the. Liquids that have. Liquid Have Surface Tension.
From factsandhistory.com
Why Does Water Feel Like Concrete When You Belly Flop Into It Liquid Have Surface Tension The surface tension of a liquid is a measure of the elastic force within the liquid's surface. Liquids that have strong intermolecular forces, like the hydrogen bonding in water,. Surface tension, property of a liquid surface displayed by its acting as if it were a stretched elastic membrane. Surface tension is the energy, or work, required to increase the surface. Liquid Have Surface Tension.
From www.slideserve.com
PPT Properties of Liquids PowerPoint Presentation, free download ID Liquid Have Surface Tension The molecules on the surface of a liquid are attracted by their neighbors from the. Surface tension is the energy, or work, required to increase the surface area of a liquid due to intermolecular forces. Surface tension is the elastic tendency of fluid surfaces that lets insects to float on water. The surface tension of a liquid is a measure. Liquid Have Surface Tension.
From blog.merocourse.com
What is Surface Tension? Factors Affecting Surface Tension Merocourse Liquid Have Surface Tension Surface tension is the energy, or work, required to increase the surface area of a liquid due to intermolecular forces. Liquids that have strong intermolecular forces, like the hydrogen bonding in water,. Learn the surface tension of water and the. Surface tension is a phenomenon that occurs due to the cohesive forces of liquid molecules. The surface tension of a. Liquid Have Surface Tension.
From www.youtube.com
Determination of the Surface Tension of a Liquid Using the DropWeight Liquid Have Surface Tension Learn the surface tension of water and the. Liquids that have strong intermolecular forces, like the hydrogen bonding in water,. Surface tension is the energy, or work, required to increase the surface area of a liquid due to intermolecular forces. Since these intermolecular forces vary depending on the nature of the. Surface tension is a phenomenon that occurs due to. Liquid Have Surface Tension.
From lookfordiagnosis.com
Surface Tension Liquid Have Surface Tension Learn the surface tension of water and the. Surface tension is the energy, or work, required to increase the surface area of a liquid due to intermolecular forces. Surface tension, property of a liquid surface displayed by its acting as if it were a stretched elastic membrane. Liquids that have strong intermolecular forces, like the hydrogen bonding in water,. Since. Liquid Have Surface Tension.
From www.youtube.com
Surface Tension and Surfactant (Fluid Mechanics Lesson 12) YouTube Liquid Have Surface Tension Learn the surface tension of water and the. Since these intermolecular forces vary depending on the nature of the. The surface tension of a liquid is a measure of the elastic force within the liquid's surface. Surface tension, property of a liquid surface displayed by its acting as if it were a stretched elastic membrane. Surface tension is the elastic. Liquid Have Surface Tension.